
Your Mission, Our Support
Working with you to bring transformative therapies to patients.
With You at Every Step
Wherever you are in the journey, we’re here with the expert guidance and support to help you move your mission forward.
Our Expertise in AAV Gene Therapy Development
Partner with our experts to define and refine your AAV therapy today.
Research-Grade Material for Proof-of-Concept Studies
We’ve helped others navigate this path and our team is here to help guide you forward.
Comprehensive Regulatory Support
Here to help shape your regulatory strategy for success and impact, with comprehensive support as you prepare for every milestone.
Proof-of-concept
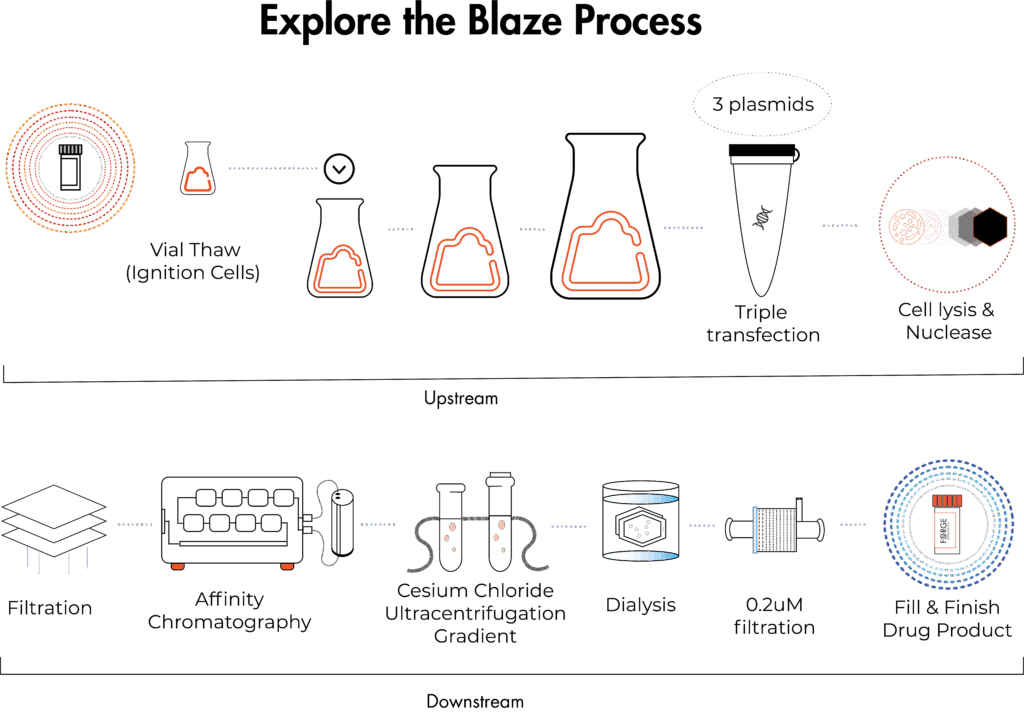
Start with Blaze Research-Grade Production
Blaze is our research-grade AAV production offering at 1–40L, designed for early-stage discovery work like generating material for proof-of-concept studies and evaluating vector manufacturability.

guiding what’s next
Support Your Next Step in AAV Production
Navigating the complexities of AAV development and manufacturing is critical to bringing effective therapies to patients. Our comprehensive manufacturing services and expert support are designed to help guide you through every step of the process.

Forge Your Path
Let’s talk about how we can help advance your therapy to patients.
Insights for Foundations
-
Christopher Shilling represented Forge and the gene therapy manufacturing sector at a recent public meeting hosted by the FDA regarding…
-
This article first appeared in Endpoints News and highlights the growing importance of scalable, reliable AAV production as gene therapy…
-
FDA PreCheck is a new initiative aimed at accelerating the establishment of drug manufacturing facilities in the U.S. through a…
-
Bringing a gene therapy to market involves navigating the FDA’s complex Biologics License Application (BLA) process, which ensures products meet…