Blaze Vector Production
We provide Blaze Vector Production to help researchers and gene therapy developers access turnkey, scalable, research-grade vector production at 1-40L without a long-term commitment.

begin with blaze
Starting with the End in Mind
Blaze leverages Forge’s proprietary, commercially viable technologies, including our HEK293 suspension Ignition Cells™, pEMBR™ 2.0 Ad helper plasmid, and modified rep/cap plasmids to enable efficient and scalable AAV production, from research through cGMP manufacturing. Developers use Blaze for:
- Generating research-grade AAV material
- Identifying lead therapeutic candidate
- Understanding the manufacturability of your vector
- Seamless scale up to cGMP
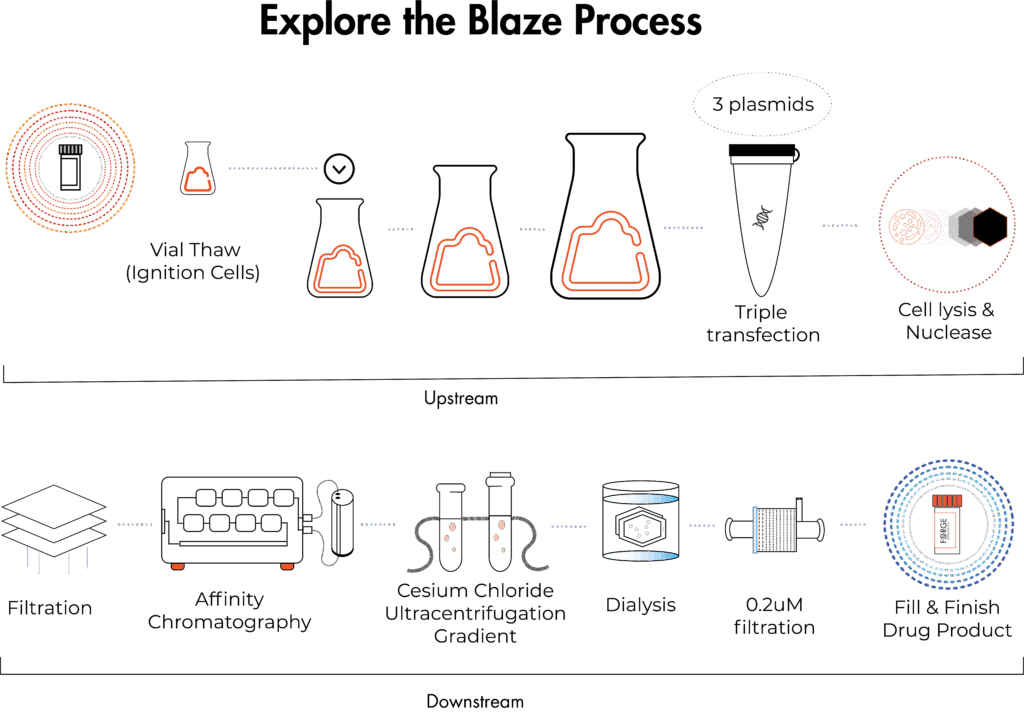
Process
How Blaze Works
Promising early data often signals the need to scale AAV production beyond what in-house capabilities can support. Blaze bridges this gap by producing research-grade AAV for proof-of-concept studies and evaluating vector manufacturability, helping developers confidently advance early-stage discovery work.
-1024x576.png)
Ready for growth
Post-Research Support
Forge provides the same expert guidance and support for your program as it moves toward clinical production. With your proof-of-concept in place, we continue to support your journey with integrated AAV manufacturing services tailored to your next phase.

Power Your Discovery with Blaze
Connect with our client development team to get started with Blaze.
Frequently Asked Questions
What is research-grade AAV used for in early-stage gene therapy development?
Research-grade AAV is used to advance early discovery and development efforts, including proof-of-concept studies, target validation, and initial assessments of vector design and manufacturability. It provides material needed to generate preclinical data and determine whether programs are ready to move forward toward larger-scale production and clinical development.
How does Blaze support proof-of-concept AAV studies?
Blaze provides fast, flexible production of research-grade AAV tailored for early-stage work. The service enables gene therapy developers to quickly obtain the material needed for proof-of-concept studies, helping evaluate therapeutic potential and make critical early decisions with confidence. Starting development with a commercially viable platform, like Blaze, can support easier development, scale-up, reduce bridging studies, and minimize risks to future approvals.
When should developers move AAV production from their lab to a CDMO?
Developers typically transition from in-house lab production to a CDMO when early data show promise and larger, more consistent batches are needed for advanced studies. This move helps ensure higher-quality materials, scalable processes, and smoother progression toward preclinical or cGMP manufacturing, reducing risks as programs advance toward regulatory milestones.
Does Forge Biologics provide AAV CRO services?
While we don’t provide traditional CRO services, like preclinical or clinical trial management, we work closely with clients to support their manufacturing strategy throughout the development lifecycle and maintain preferred partnerships with CROs that we can connect our clients to as needed.
Insights for Developers
-
This article first appeared in Endpoints News and highlights the growing importance of scalable, reliable AAV production as gene therapy…
-
Bringing a gene therapy to market involves navigating the FDA’s complex Biologics License Application (BLA) process, which ensures products meet…
-
23 Questions with Forge is a fast-paced series where team members answer rapid-fire questions on trending topics in gene therapy…
-
Adeno-associated virus (AAV) gene therapies hold transformative potential to treat genetic disorders at their source, but their complexity demands rigorous…