Blaze Vector Production
We provide Blaze Vector Production to help researchers and gene therapy developers access turnkey, scalable, research-grade vector production at 1-40L without a long-term commitment.

begin with blaze
Starting with the End in Mind
Blaze leverages Forge’s proprietary, commercially viable technologies, including our HEK293 suspension Ignition Cells™, pEMBR™ 2.0 Ad helper plasmid, and modified rep/cap plasmids to enable efficient and scalable AAV production, from research through cGMP manufacturing. Developers use Blaze for:
- Generating research-grade AAV material
- Identifying lead therapeutic candidate
- Understanding the manufacturability of your vector
- Seamless scale up to cGMP
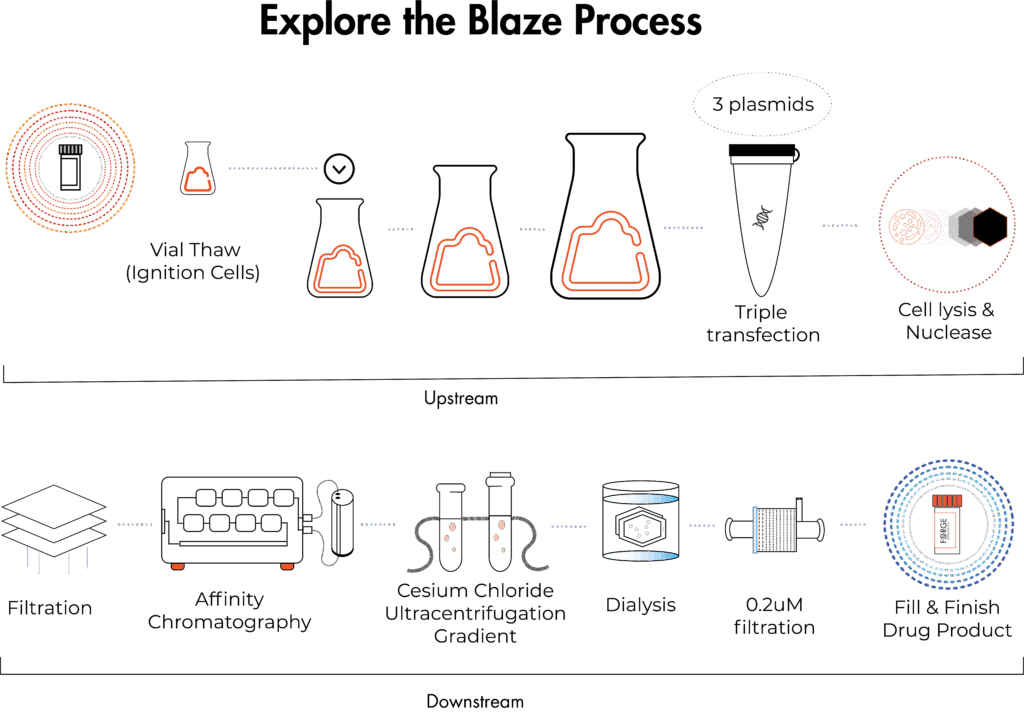
Process
How Blaze Works
Promising early data often signals the need to scale AAV production beyond what in-house capabilities can support. Blaze bridges this gap by producing research-grade AAV for proof-of-concept studies and evaluating vector manufacturability, helping developers confidently advance early-stage discovery work.
-1024x576.png)
Ready for growth
Post-Research Support
Forge provides the same expert guidance and support for your program as it moves toward clinical production. With your proof-of-concept in place, we continue to support your journey with integrated AAV manufacturing services tailored to your next phase.

Power Your Discovery with Blaze
Connect with our client development team to get started with Blaze.
Frequently Asked Questions
Does Forge Biologics provide AAV CRO services?
Forge Biologics is a CDMO (Contract Development and Manufacturing Organization) and does not provide CRO services. We specialize in AAV manufacturing, offering research-grade production, process development, GMP manufacturing, and integrated support services such as molecular development, analytics, and regulatory consultation. While we don’t provide traditional CRO services—like preclinical or clinical trial management—we work closely with clients to support their manufacturing strategy throughout the development lifecycle and maintain preferred partnerships with CROs that we can connect our clients to as needed.
How quickly can Forge deliver a GMP batch?
Timelines for GMP batch delivery are highly dependent on the specifics of your program, including vector design, process readiness, and scope of work. By partnering with Forge and leveraging our proprietary platform and integrated services—such as in-house analytics and plasmid production—developers may accelerate timelines to GMP manufacturing by up to 30% compared to full process development. Connect with our Client Development team to discuss your program and receive a more detailed scope of work based on your program needs.
Hot of the Forge
-
Fractyl Health will leverage Forge’s FUEL™ platform to manufacture AAV for Rejuva, Fractyl’s pancreatic gene therapy platform for patients with…
-
Oral and poster presentations will highlight data demonstrating the enhanced productivity, recovery, and consistent quality of Forge’s FUEL™ AAV manufacturing…
-
“We’re proud to support Affinia’s vision of bringing hope to patients affected by cardiomyopathy through their innovative gene therapy.”
-
FUEL™ platform can achieve a 2-6x increase in productivity compared to industry standard, and includes pEMBR 2.0™ Ad helper and…