Innovative AAV Manufacturing
The FUEL™ Platform
As gene therapy developers push the boundaries of innovation, manufacturing must keep pace. Forge’s FUEL™ platform delivers a more efficient manufacturing foundation through advanced technologies, proven processes, and product-specific optimizations that help developers deliver more doses from the same run.
fuel progress
Get More from Every Run with the FUEL™ Platform
By leveraging a manufacturing platform that combines increased productivity with product-specific optimizations, you gain more from every run while maintaining exceptional quality.
advanced technology
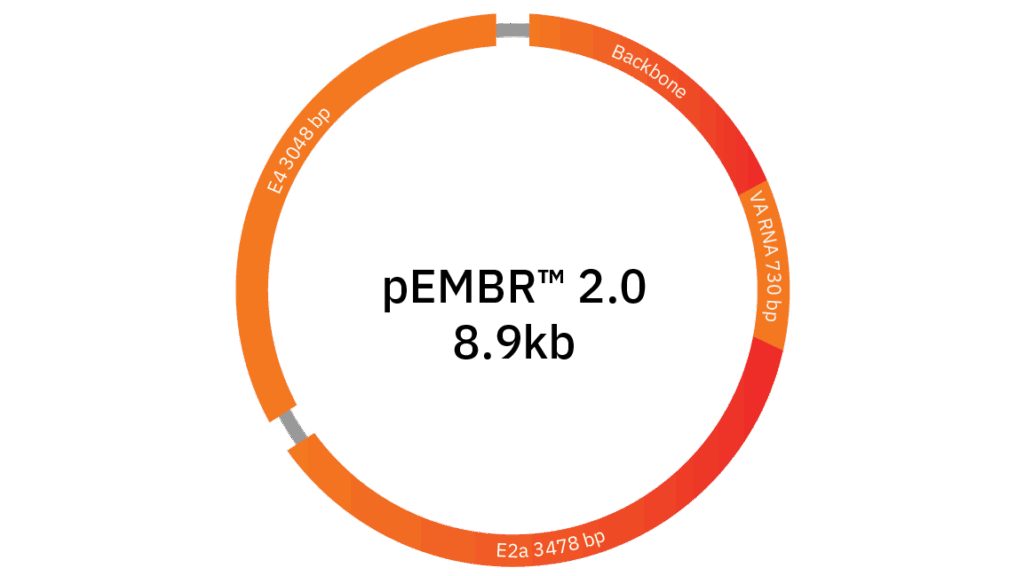
pEMBR™ 2.0 Ad Helper
As one of the smallest Ad helpers commercially available at 8.9 kb, pEMBR™ 2.0 increases manufacturing efficiency, offers an improved safety profile by reducing unnecessary adenoviral components for AAV production, and is Forge-specific intellectual property.

advanced technology
Modified Rep/Caps
Designed for safety and higher AAV yields, Forge’s patent pending modified rep/cap incorporates the client-specific capsid sequence into its proprietary backbone plasmid.

advanced technology
Ignition Cells™
Our suspension HEK293 Ignition Cells™ are optimized for robust transient transfection and can achieve over 90% full capsids post-enrichment, often with undetectable particles. Ignition Cells™ are supported by fully qualified Master Cell Bank and Working Cell Bank.
Novel Capsid? Yeah, Our Platform Can Work With That
The FUEL™ platform is designed to support all serotypes, including novel capsids.
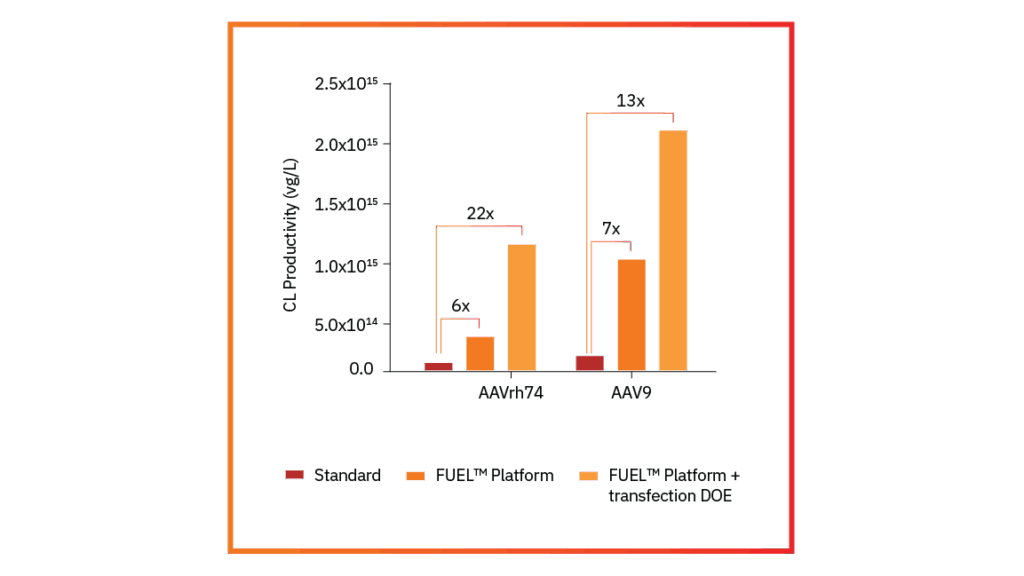
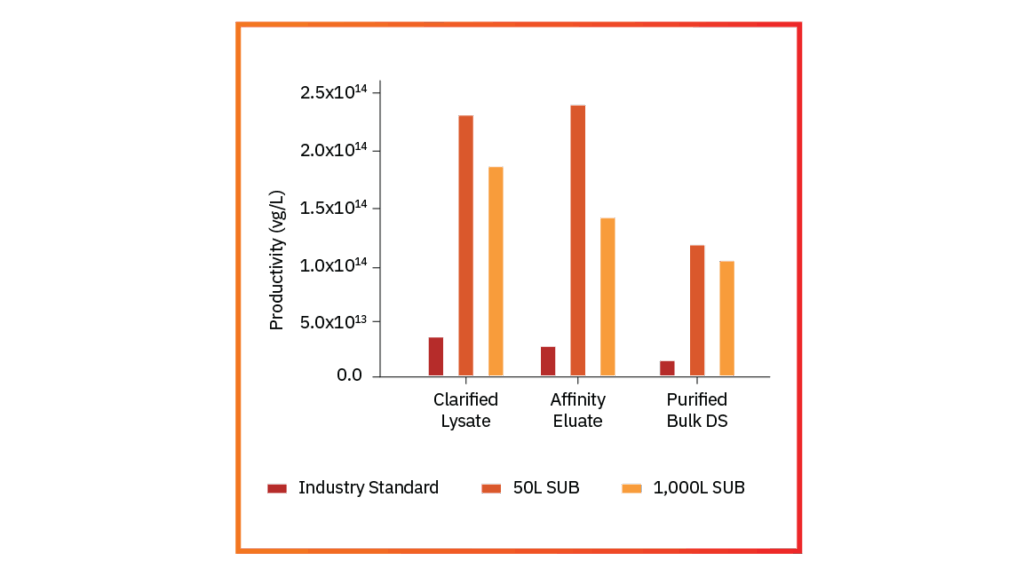
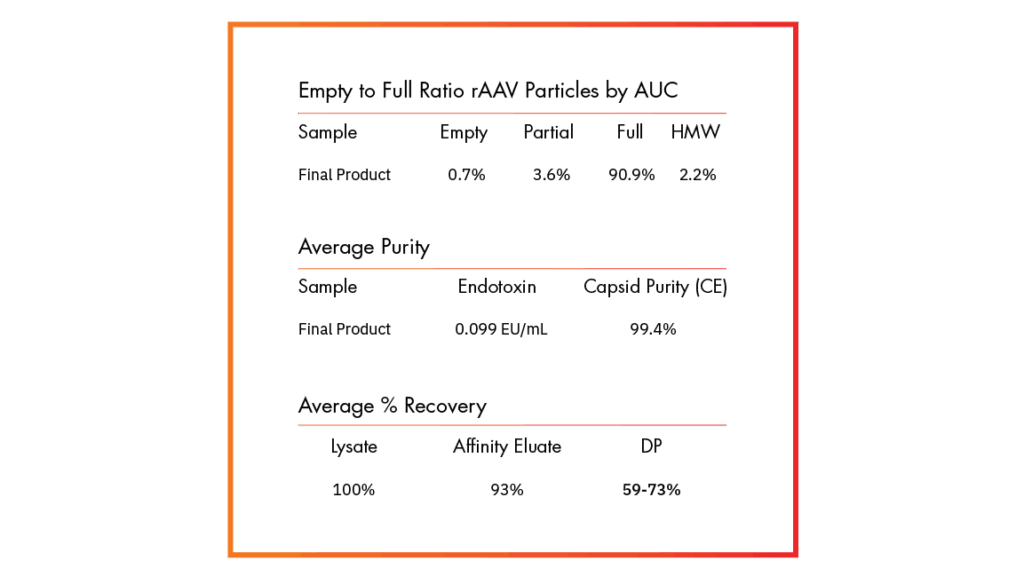
Higher Yield, Better Recovery, Same Unmatched Quality
Explore how our FUEL™ platform boosts productivity, scales seamlessly, improves recovery, and maintains consistent quality.
Product-Specific Optimizations
The FUEL™ platform offers a strong foundation for further enhancements, combining platform technologies with product-specific optimizations to help you achieve high yield, consistent quality, and reliable performance. Here are some of the optimizations we’ve used to help developers achieve their goals.
Yield Optimization
Downstream Scalability
Downstream Recovery

Ready to Fuel Your Program?
Let’s talk about how we can help accelerate your therapy to patients.
Frequently Asked Questions
How does the FUEL™ platform contribute to more efficient and scalable manufacturing?
The FUEL™ platform has the potential to deliver higher AAV yields per liter, allowing developers to achieve their required clinical vector quantities with a substantial decrease in bioreactor volume.
What is Forge’s FUEL™ platform, and how does it support AAV manufacturing?
The FUEL™ platform is Forge’s advanced AAV manufacturing solution designed to increase yields, improve scalability, and enhance efficiency. It combines Forge’s proprietary technologies, proven manufacturing processes, and product-specific optimizations to help gene therapy developers bring more therapies to more patients
Is the FUEL™ platform suitable for pre-clinical to commercial manufacturing?
Yes. The FUEL™ platform uses commercially viable technologies and processes designed to seamlessly scale from early-stage AAV manufacturing through to commercial production.
Can the FUEL™ platform be customized for my specific AAV serotype or program needs?
Absolutely. The FUEL™ AAV platform is designed to support all serotypes, including novel capsids.
What production scales can the FUEL™ platform accomodate?
The FUEL™ AAV platform supports manufacturing from small-scale research batches up to large-scale clinical production, with proven scalability from 1L to 1,000L.
Insights for Developers
-
This article first appeared in Endpoints News and highlights the growing importance of scalable, reliable AAV production as gene therapy…
-
This article, first published in Endpoints News, explores how innovative manufacturing approaches are helping gene therapy developers overcome long-standing production…
-
Gene therapy manufacturing is complex and time-sensitive, but Forge’s FUEL™ AAV platform was built to accelerate and optimize production through…
-
In this episode of 23 Questions with Forge, Senior Manager of Technical Sales Victoria Maharaj spotlights Forge Biologics’ groundbreaking FUEL™…