
Your Research, Realized.
Transform your early research into manufacturing readiness through expert guidance built for your stage.
Built to Move You from Discovery to Development
Early-phase programs deserve more than a starting point. Here’s how we help set you up for long-term success.
Research-Grade Material for Proof-of-Concept Studies
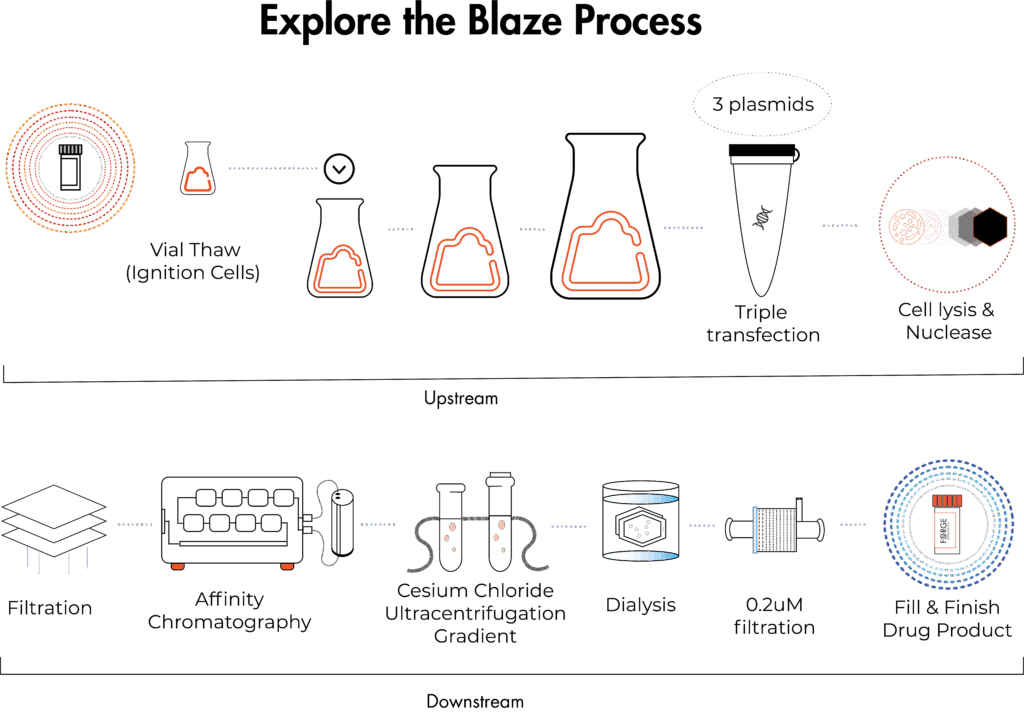
Blaze, our research-grade manufacturing offering, helps generate material for proof-of-concept studies, giving you insight into the manufacturability of your vector.
A Platform Built to Take You from Concept to Commercial
Utilizing the commercially viable FUEL™ platform supports easier development and scale-up, reduces bridging studies, and minimizes risks to future approvals.
Robust Early Analytics that Build a Stronger Foundation
Partner with our experts to define and refine your AAV quality attributes early.
Proof-of-concept
Start with Blaze Research-Grade Production
Blaze is our research-grade AAV production offering at 1–40L, designed for early-stage discovery work like generating material for proof-of-concept studies and evaluating vector manufacturability.

Ready for growth
Advancing Beyond Discovery
Forge provides the same expert guidance and support for your program as it moves toward clinical production. With your proof-of-concept in place, we continue to support your journey with integrated AAV manufacturing services tailored to your next phase.

Forge Your Path
Let’s talk about how we can scale your AAV research today.
Insights for Researchers
-
Christopher Shilling represented Forge and the gene therapy manufacturing sector at a recent public meeting hosted by the FDA regarding…
-
This article first appeared in Endpoints News and highlights the growing importance of scalable, reliable AAV production as gene therapy…
-
Bringing a gene therapy to market involves navigating the FDA’s complex Biologics License Application (BLA) process, which ensures products meet…
-
23 Questions with Forge is our fast-paced series where our team members are challenged to answer 23 rapid-fire questions about…
